Purpose
Today's laboratory will begin to develop the principles of microscale synthesis. Microscale techniques are those methods which usually work with less than 1 gram of reagent.
There are numerous benefits to working on this small scale. First, the procedure is usually much quicker since any step which involves heating, cooling, dropwise addition, etc. will take place much more rapidly when using these small quantities than when using larger quantities. Second, the lab environment is safer since smaller quantities of materials are in use. Air quality in the laboratory as well as danger from spills is much improved at this scale. Third, there is an economic advantage to working at a small scale. Initial costs of chemicals are actually somewhat higher when using microscale. For example, a recent Aldrich Chemical Company catalog reports bromobenzene's price as $8.70 for 100 mL vs. $76.10 for 2000 mL. Clearly, a price advantage is obtained when buying larger quantities. For a research lab, though, this benefit is quickly diminished when considering the high cost of chemical waste disposal. It is clearly economically and environmentally advantageous to perform research at a smaller scale. Fourth, small amounts which might be discarded in larger preparations are now important when working with milligram yields. A chemist who is working at this scale must quickly learn to use skill and care in the lab.
The microscale lab to be performed is the Grignard synthesis of a 3° alcohol from a ketone.
Step 1 - Formation of Grignard Reagent
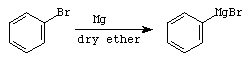

Note that since all three of the alkyl groups on this product are the same (phenyl) it would also be possible to prepare this product by a Grignard reaction with an appropriate ester.
Procedure
Into a clean DRY 18 x 150mm test tube, weigh 85 mg (+/- 5 mg) of magnesium, then add about 1 mL of anhydrous ether (WARNING: Ether - NO FLAMES!) to the test tube using a pipet. Into a micro-test tube, measure 0.35 mL of bromobenzene from a buret. Add about about half of the bromobenzene to the magnesium/ether mixture using a pipet. Dissolve the remaining bromobenzene in about 1 mL of ether. Carefully crush the magnesium in the test tube with a clean dry stirring rod. Exercise caution to avoid poking the bottom out of the test tube. Watch for the
reaction to begin, evidenced by noticeable bubbling which occurs in the solution. The
solution will also become cloudy as the reaction proceeds. If no reaction is observed after
about five minutes, crush the magnesium again.
Once the reaction is underway, add 5 drop portions of the bromobenzene solution at 30 to 60 second intervals. After all of the bromobenzene has been added, rinse the micro-test tube with about 1 mL of ether, adding the ether rinse to the reaction mixture in one portion. Wait for the reflux to stop before continuing.
Pre-lab questions:
1. What does "reflux" mean?
2. What was the purpose of pouring the ether rinse into the
test tube rather than into the organic waste bottle?
While waiting for the reflux to stop, prepare a solution of 300 mg of benzophenone in 1 mL of ether in a micro-test tube. Gentle swirling will help to dissolve the ketone. Once the Grignard mixture has stopped refluxing, begin adding the ketone solution dropwise. Add about 5 drops every thirty seconds. Swirl after each addition. After all of the ketone has been added rinse the micro-test tube with about 1 mL of ether adding the rinse to the reaction mixture in one portion.
Cool to reaction mixture to room temperature in a cool water bath. Add 5 mL dilute HCl (DROPWISE AT FIRST) while swirling in the water bath. Transfer the reaction mixture to a 25 x 100mm test tube. Rinse the reaction tube with 2 mL ether and 2 mL 6 M HCl combining these rinses with the reaction mixture. Agitate the mixture and allow the layers to separate. Remove the aqueous layer with a pipet.
Pre-lab question:
How many millimoles of benzophenone are to be used?
Pre-lab questions:
1. What does "aqueous" mean?
2. What is the density of ether?
3. Which layer is the aqueous layer in this procedure?
Dry the ether solution over sodium sulfate for 5 to 10 minutes, then filter the solution through a cotton plug into a 50 mL beaker. Evaporate the ether over a steam bath, aspirating the ether vapors with a water aspirator. At this point the crude product will be present as an oily layer at the bottom of the beaker (occasionally crsytals may be observed). Add about 5 mL of pentane or hexane to the crude product. Stir for several minutes to crystallize the product. Remove the solvent from the product by using a pipet which is touching the bottom of the beaker.
Pre-lab question:
The product of this reaction is very soluble in ether but
not at all soluble in pentane or hexane. Explain.
Dry the sample on a watch glass until the next class. Record mass of the product and determine the melting point in duplicate. Calculate limiting reagent and percentage yield.
Alternate methods
You may use the original amount of magnesium, bromobenzene and ether and then replace the benzophenone
with an equal number of millimoles of an ester, such as methyl benzoate or ethyl benzoate. Note that the
ester will be present in a large excess since esters react with two equivalents of Grignard reagent.
Pre-lab questions:
1. What is the molecular weight and density of methyl benzoate?
2. What volume (in microliters) of methyl benzoate could be used to replace benzophenone?
3. What is the molecular weight and density of ethyl benzoate?
4. What volume (in microliters) of ethyl benzoate could be used to replace benzophenone?
References
The Grignard Reaction
Nobel Prize, 1912
Calculating limiting reagents and percentage yield
Aldrich Chemical Company Search Page
ChemExper Chemical Directory
Acros Chemicals
NIST Chemistry Webbook