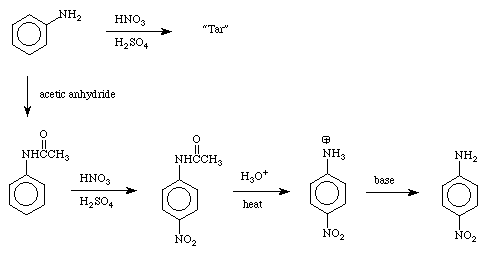

Procedure
In a 125-mL Erlenmeyer flask, place 2.7 g of acetanilide (which was prepared in laboratory last semester). If you have more than 2.7 g, simply use 2.7 g as directed and save the excess. If you have less than 2.7 g
but more than 1.5 g, proceed as directed; however, reduce the amount of nitric acid, below, by an
appropriate percentage. In other words, if you have 2.1 g multiply the quantity by a factor of (2.1 divided
by 2.7). If you have less than 1.5 g, see your instructor.
Obtain 9 mL of concentrated sulfuric acid and pour about half of the acid into the flask. Swirl and stir the
mixture until all but traces of the acetanilide dissolve (a small amount of remaining solid will subsequently
dissolve). Cool the solution in an ice bath. With a buret measure out 1.5 mL of concentrated nitric acid and
add it to the remaining sulfuric acid. Mix the acids by drawing up samples from the bottom with your
disposable pipet and bulb and emptying them onto top; complete the mixing by discharging a stream of
bubbles from the empty pipet at the bottom of the cylinder.
Using the pipet and bulb, add the mixed acid in small portions (about 0.5 mL) to the cooled sulfuric acid
solution of acetanilide. Swirl the flask in the ice bath after each portion is added. The flask should not
become perceptibly warm to the touch; the addition requires 10 to 15 minutes. After 20 minutes, including
addition time, add 25 mL of a mixture of ice and water to the reaction mixture.
The resulting suspension of nitroacetanilides is now hydrolyzed in the same flask, using the aqueous sulfuric acid. Add a boiling stone and heat the flask on a hot plate. Watch carefully as the color darkens and the solid begins to dissolve, and remove the heat source if necessary to avoid excessively vigorous boiling and foaming CAUTION!!! This will boil over if you are not extremely careful!!!
Continue heating at a gentle boil for 15 minutes and then cool the flask and contents in an ice bath. Place the bath and flask in the hood and add, in five or six portions, 25 mL of concentrated aqueous ammonium hydroxide; swirl in the ice bath after each addition.
Collect the precipitated nitroaniline in a Büchner funnel by suction filtration. Rinse the flask with 3 mL of water, disconnect the suction tubing, pour the rinsing into the funnel, and then suck the water through the precipitate; press the solid, and allow air to be drawn through for 2 or 3 minutes. DISPOSE OF THE FILTRATE IN THE AQUEOUS WASTE.
We will now purify the product by recrystallization from water. Several bits of information must be obtained to do this. First, calculate the theoretical yield of the reaction (assuming 2.7 g acetanilide). Assume that all three steps from acetanilide to para-nitroaniline occur with 100% yield.
Theoretical yield = ____________ g
Next, multiply your result by the scaledown factor from the first paragraph. If you used 2.7 g of acetanilide, your result will be the same as the previous result.
Scaled theoretical yield = ____________ g
Next, according to the Merck Index, 1 g of para-nitroaniline dissolves in 45 mL of boiling water. However, para-nitroaniline is about 30 times less soluble in room temperature water. It is less soluble still in ice cold water. Hence, water can be used for recrystallization. How many milliliters of water would be required to dissolve the para-nitroaniline if your yield is the same as in the previous result?
Solvent required = ____________ mL
What size beaker would be best to choose for boiling the volume of water which you calculated in the previous result?
Beaker required = ____________ mL
Transfer the yellow crystals, which can be peeled cleanly from the filter paper, to a beaker of the proper size. Add the volume of water which you calculated, place a watch glass over the top of the beaker to prevent evaporation and heat slowly on a hot plate until it comes to a gentle boil. Heat and stir the mixture until most of the solid dissolves. If many crystals seem to be undissolved, add more water in 4-5 mL portions. Do not add more than 25% additional water, compared to your original estimate. Occasionally a few very deeply colored crystals will resist solution.
Next cool in an ice bath for 10 to 15 minutes to crystallize the p-nitroaniline. Collect the crystals by suction filtration. DO NOT DISPOSE OF THE FILTRATE which is now described as the "mother liquor" from which the crystals were harvested. Transfer the crystals and filter paper to dry on a labelled watch glass.
Transfer the mother liquor to an appropriate beaker and boil gently to reduce the volume to about one-tenth
the original volume. Label the beaker, parafilm the top, place a watch glass over the parafilm and leave in
your locker until next class. At that time, cool in an ice bath and stir to obtain a second crop of crystals.
Filter with a small Büchner funnel. Dispose of the filtrate in the AQUEOUS waste container.
Determine melting point and mass of both the first crop and second crop of crystals. Calculate percentage yield by combining the mass of the two crops.